Pharmaceutical repackaging has become a common practice in the healthcare industry, offering numerous benefits such as improving patient adherence. This article, tailored for distributors, delves into the various aspects of pharmaceutical repackaging and its potential to transform the distribution landscape.
As of November 27, 2019, the Drug Supply Chain Security Act (DSCSA) set out requirements for the labelling of products at the package level. The guidance for the industry on product identifiers defines a package as:
The “smallest individual saleable unit of product for distribution by a manufacturer or re-packager that is intended by the manufacturer for ultimate sale to the dispenser of such product.” For purposes of this definition, an individual saleable unit is defined under section 581(11)(B) of the FD&C Act as the “smallest container of product introduced into commerce by the manufacturer or re-packager that is intended by the manufacturer or re-packager for individual sale to a dispenser.”
According to this definition, the manufacturer's smallest product container designed for individual sale to a dispenser is classified as an individual saleable unit. For instance, a drug manufacturer producing individual vials could opt to designate a tray of 25 vials as the smallest saleable unit, as illustrated in this real-world example:
In this example, the tray containing 25 vials is considered as one unit with the assigned serial number. However, the individual vials in the tray are not considered as the smallest individual saleable unit by the manufacturer and hence are not serialized. Therefore, they cannot be transacted under the DSCSA regulations.
If the product is being distributed to larger pharmaceutical distributors or pharmacies selling large quantities of these products, this may not be an issue. However, secondary or independent distributors who purchase the product by the case and want to resell the product to smaller pharmacies, clinics, or doctor's offices may face an issue. According to the DSCSA regulations, a pharmaceutical distributor cannot "break the case" and sell only one of the individual units as there is no serial number on the individual unit.
What is Pharmaceutical Repackaging?
Pharmaceutical repackaging involves the process of taking bulk quantities of medications and repackaging them into smaller, more manageable units. This practice is not only crucial for hospitals and pharmacies but also for distributors selling in eaches, who need to provide medications in specific doses or quantities as required by healthcare providers.
In the above example, a small clinic may not be able to use 25 vials of a drug before the product expires. Thus, if forced to purchase in that quantity, the result could be wasted product and higher healthcare costs. This is because the cost of the full tray of 25 vials needs to be recovered even if only 1 or 2 vials from that tray would otherwise be used.
The Role of the Distributor
Distributors play a pivotal role in the repackaging ecosystem. They bridge the gap between manufacturers and healthcare providers, ensuring that medications are delivered safely and efficiently. Pharmaceutical distribution companies are increasingly adopting repackaging services to add value to their offerings and differentiate themselves in a competitive market.
While pharmaceutical repackaging presents numerous possibilities, it also comes with challenges. Regulatory compliance is a major concern, with stringent guidelines governing the repackaging process to ensure patient safety. Distributors must invest in quality control and traceability systems to maintain the integrity of the medications throughout the supply chain and this presents a business opportunity to enterprising individuals who are willing to take on the challenge of re-labelling the eaches using their own company prefix, NDC and GTIN for the individual vials.
How to Repackage Pharmaceutical Product
So, how can distributors who deal with smaller pharmacies and clinics on a daily basis solve the problem of pharmaceutical repackaging/relabeling of products?
It sounds easy, break the case, print new labels with new serial numbers and stick them on the individual units, right? Wrong! Only companies that have special licenses with specific “certified” software can legally repackage and relabel products.
Therefore, a distributor must find a service provider, such as a re-packager or CMO (Contract Manufacturing Organization), to perform the re-labeling process. This is not as easy as simply adding a new serial number on the individual vial, for example. The service provider is required to re-number the product(s) with their own company identifier to indicate that they performed the re-packaging/relabeling.
Of course, this process comes with a cost that must be taken into consideration when the distributor is selling individual units to smaller pharmacies, clinics, doctor's offices, and other similar establishments.
Transforming a Case to Eaches
Let’s consider the physical and financial processes that need to be undertaken to transform a case of product (containing only a Serial Number at the case level) into individual units. We will assume for this scenario that the pharmaceutical distributor has already purchased the case of product “Product-A” and it only contains a serial number on the outside of the case.
Manual Process of Adjusting Inventory and Costs:
- “Product-A” is received into the distributor’s inventory as a case
- A purchase order for the cost of the labor required to perform the repackaging is issued to the re-packager
- In the warehouse, staff pick a case, making note of the lot number to be removed from the inventory
- Inventory adjustment is then required to remove the case from inventory, making note of the cost of the case of inventory
- Re-packager sends the now re-packaged ‘eaches’ to the distributor as a new product, labelled with a new NDC and GTIN
- Note: If the ‘eaches’ produced were packed into a new sealed case, the new sealed case may also be assigned a distinct GTIN and serial number
- Inventory of the now repackaged product has to be adjusted up at the unit level, based on the pro-rated cost of the case, plus the fees charged by the re-packager
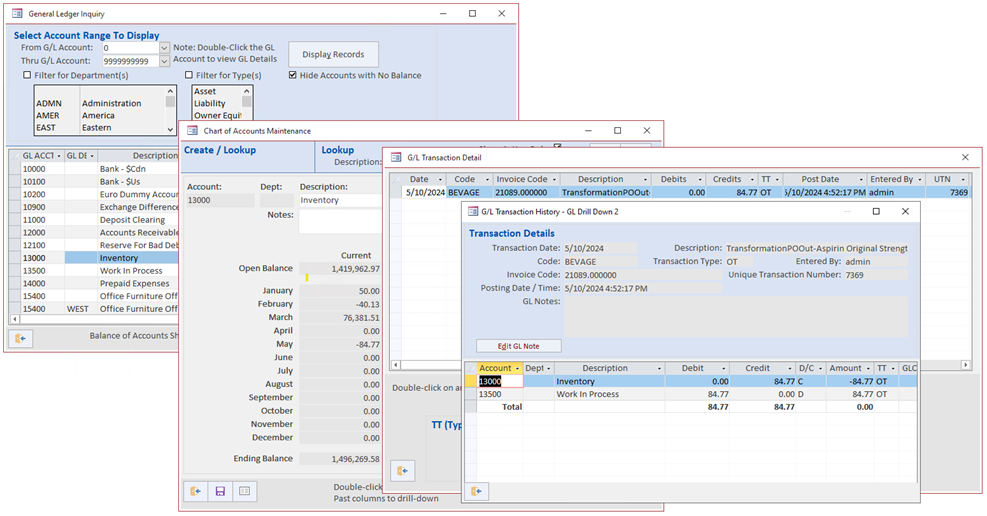
Blue Link Pharmaceutical ERP Transformation Process
Using the Blue Link Pharmaceutical traceability software, the process of transforming a case into eaches is still required. However, there is built-in functionality to manage the entire process:
- Create a Transformation PO
- Allows users to provide instructions to the re-packager on what you want to do
- Allows the users to assign the special lot number of the case being sent
- A picking slip can be generated to instruct warehouse staff what specific quantity of which lot to pick to send to the re-packager
- The transformation PO ship step automatically adjusts out of inventory the case quantity along with the associated cost
- The cost of the transformed products inbound (at the ‘each’ level) will automatically incorporate both the cost of the case sent for re-labelling, but also the cost of the service being performed

- The product is received in the same manner as any product
- No adjustments are required in inventory or accounting
- The cost associated with the received product includes the initial cost for the case of the product and the cost to re-label the individual units
Assuming that you receive an EPCIS file from the re-packager, containing the serial numbers of the sealed case and case contents, the process for receiving the inbound repackaged items will be the same as the receipt process for any other inbound products sent by other manufacturers or distributors: scan the 2D data matrix barcode on the outside of the case, which will be electronically compared to the EPCIS file sent by the supplier.
Now, you will have a new version of the original drug available for sale at the ‘each’ level. For example, if you had a tray of 25 vials previously, it could now come back into inventory as individual vials under a new NDC/GTIN. This allows you to meet the smaller volume requirements of smaller clinics or physicians who only need a small quantity.
This article references this FDA guidance: Guidance for Industry Standards for Securing the Drug Supply Chain - Standardized Numerical Identification for Prescription Drug Packages
Discover how Blue Link effectively monitors serialized inventory in the webinar provided below:












